Myelodysplastic syndromes (MDS) are heterogeneous clonal bone marrow neoplasms characterised by marrow dysplasia, low blood counts and risk of progression to acute myeloid leukaemia (AML).
Hypomethylating agents (HMAs) decitabine and azacitidine (AZA) are effective treatments for hematologic cancers including MDS.
AZA is indicated to treat International Prognostic Scoring System (IPPS) defined Intermediate-2 (Int-2) and high-risk (HR) MDS, low blast AML (LB-AML; 20-30% blasts) with multi-lineage dysplasia, and chronic myelomonocytic leukaemia (CMML) with 10-29% blasts without myeloproliferative disorder.
Oral decitabine/cedazuridine is currently the only oral hypomethylating agent indicated to treat IPSS Int-1, Int-2 or HR MDS and CMML, in the United States, Canada and Australia.
AZA administration requires daily visits to a hospital or clinic for 7 days every 28 days, for either intravenous infusions, or large-volume subcutaneous (SC) injections for a minimum of 6 cycles.This places a burden on this patient population, resulting in low treatment adherence, early discontinuation, and subsequently low treatment uptake.
Oral decitabine/cedazuridine is administered as one tablet daily for the first 5 days of a 28-day cycle for a minimum of 4 cycles. This allows patients to take their medication at a location of their choice.
Alongside previously reported preference for oral therapy, patients may favour oral decitabine/cedazuridine over SC AZA due to improved treatment convenience and reduced discomfort. However, this is untested in a controlled setting. This study aims to evaluate preference between oral decitabine/cedazuridine and SC AZA treatment for adult patients with Revised International Prognostic Scoring System (IPSS-R) intermediate, IPSS Int-2 or HR MDS, LB-AML or CMML.
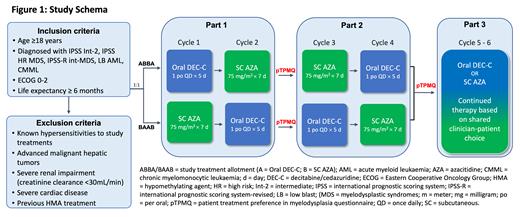
This phase 3b, open-label, multi-centre study (NCT05883956) has sites planned in Australia (4 sites) and New Zealand (5 sites), to compare preference for oral decitabine/cedazuridine (Treatment A) and SC AZA (Treatment B). The study design includes 28 days of screening, four continuous 28-day cycles of study treatment, and a follow-up period with two 28-day cycles of continued therapy. Patients will be randomised to two balanced treatment sequences: ABBA or BAAB (Figure 1). Patients will express a preference twice in the study; after completing Cycle 1 and 2, and after completing Cycle 3 and 4. This cross-over design is twice the length of a typical 2-period AB vs BA design and allows patients to act as their own controls. The study design allows estimation of the treatment effect while mitigating factors that may influence preference. These include the order of treatments, and fluctuations in the patient's physical and disease state over the course of the trial. All available preferences expressed throughout the trial will be used for primary analysis. A total of 42 patients are planned for this study.
The primary objective of this study is to compare patients' treatment preference for oral decitabine/cedazuridine and SC AZA for the treatment of MDS, LB-AML or CMML. Secondary objectives include evaluation of carers' and clinicians' treatment preference between oral decitabine/cedazuridine and SC AZA for the treatment and continued treatment of adult patients with MDS, LB-AML or CMML.
The primary objective will be assessed by a treatment preference in myelodysplasia questionnaire (pTPMQ), which instructs patients to indicate a preference for injection, oral/tablets or no preference. The secondary objectives will be evaluated by a carer treatment preference in myelodysplasia questionnaire (cTPMQ) and a medical clinician treatment preference in myelodysplasia questionnaire (mTPMQ).
Treatment discontinuation rates, quality of life and safety of SC AZA and oral decitabine/cedazuridine will also be compared.
The primary analysis will be conducted with a multilevel logistic regression model analysing all available preferences. Descriptive statistics including frequencies, proportions, mean, standard deviation, median, and interquartile range will be used to summarize the study data.
When complete, this study will address an evidence gap in the comparison of patient preference for oral decitabine/cedazuridine and SC AZA, and subsequently inform the value of oral decitabine/cedazuridine for the treatment of MDS, LB-AML and CMML.
OffLabel Disclosure:
Enjeti:Jazz: Honoraria; RACE oncology: Honoraria; Pfizer: Honoraria; Otsuka: Honoraria, Speakers Bureau; Servier: Honoraria; Astellas: Honoraria; AbbVie: Honoraria, Speakers Bureau. Fong:BeiGene: Honoraria; Jazz: Honoraria; BMS: Honoraria; Astellas: Honoraria; Amgen: Honoraria; AbbVie: Honoraria, Speakers Bureau; Servier: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Otsuka: Honoraria; Novartis: Honoraria. Castaldi:Otsuka Australia Pharmaceutical Pty Ltd: Current Employment. van Wyk:IQVIA Biotech NZ: Current Employment. Walton:RJ Labs: Current Employment; Otsuka: Consultancy. Paine:Otsuka Australia Pharmaceutical Pty Ltd: Current Employment. Keer:Astex Pharmaceuticals, Inc.: Current Employment.
Azacitidine is not indicated for patients with IPSS intermediate 1 or CMML patient with blast counts of less than 10%.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal